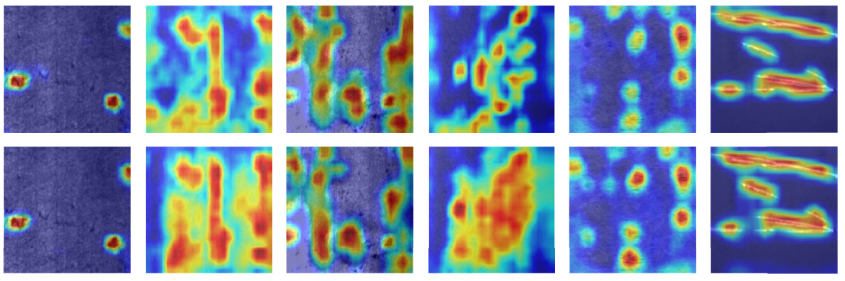
This article resolutely uses the concept of feature fusion to establish a deep learning model that can quickly recognize objects and complete an anti-counterfeit label recognition system. The receiver combines the training of the technology acceptance model (TAM) to evaluate the satisfaction of users in completing the anti-counterfeit label classification training. In this study, the fusion-based recognition program was employed to extract the feature sets of different categories of anti-counterfeit labels based on the operation of multilayer convolutional neural networks (CNNs) with different depth models. Using neighborhood components analysis, ten important sets of features from different CNN models were selected and reorganized parallelly into a new small-scale feature fusion dataset. By using naive Bayes and support vector machine methods, efficient classification of wine label image feature datasets after fusion was achieved. The feature fusion anti-counterfeiting label recognition system proposed in this article had a maximum recognition accuracy of 99.29% and a data reduction compression ratio of about 1/50. In addition to reducing training time, it maintained a high level of accuracy. This study established a TAM with the advantage of a feature fusion anti-counterfeit label recognition system. The model was tested on 100 consumers, and a satisfaction evaluation and validation analysis with partial least squares structural equation modeling were completed thereafter. The efficiency of the fusion-based deep learning model met the level of consumer satisfaction. This will be beneficial for educating consumers to use and enhance their willingness to promote and repurchase wine products in the future.
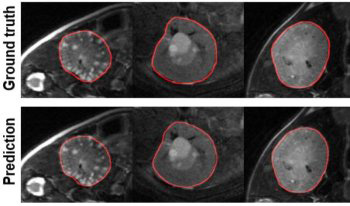
The aim of this work is to transfer the model trained on magnetic resonance images of human autosomal dominant polycystic kidney disease (ADPKD) to rat and mouse ADPKD models. A dataset of 756 MRI images of ADPKD kidneys was employed to train a modified UNet3+ architecture, which incorporated residual layers, switchable normalization, and concatenated skip connections for kidney and cyst segmentation tasks. The trained model was then subjected to transfer learning (TL) using data from two commonly utilized animal PKD models: the Pkdh1pck (PCK) rat and the Pkd1RC∕RC (RC) mouse. Transfer learning achieved Dice similarity coefficients of 0.93±0.04 and 0.63±0.16 (mean±SD) for a sample combination of PCK+RC kidneys and cysts, respectively, on the test datasets of animal images. We showcased the utilization of TL in situations involving constrained source and target datasets and have achieved good accuracy in the cases of class imbalance.