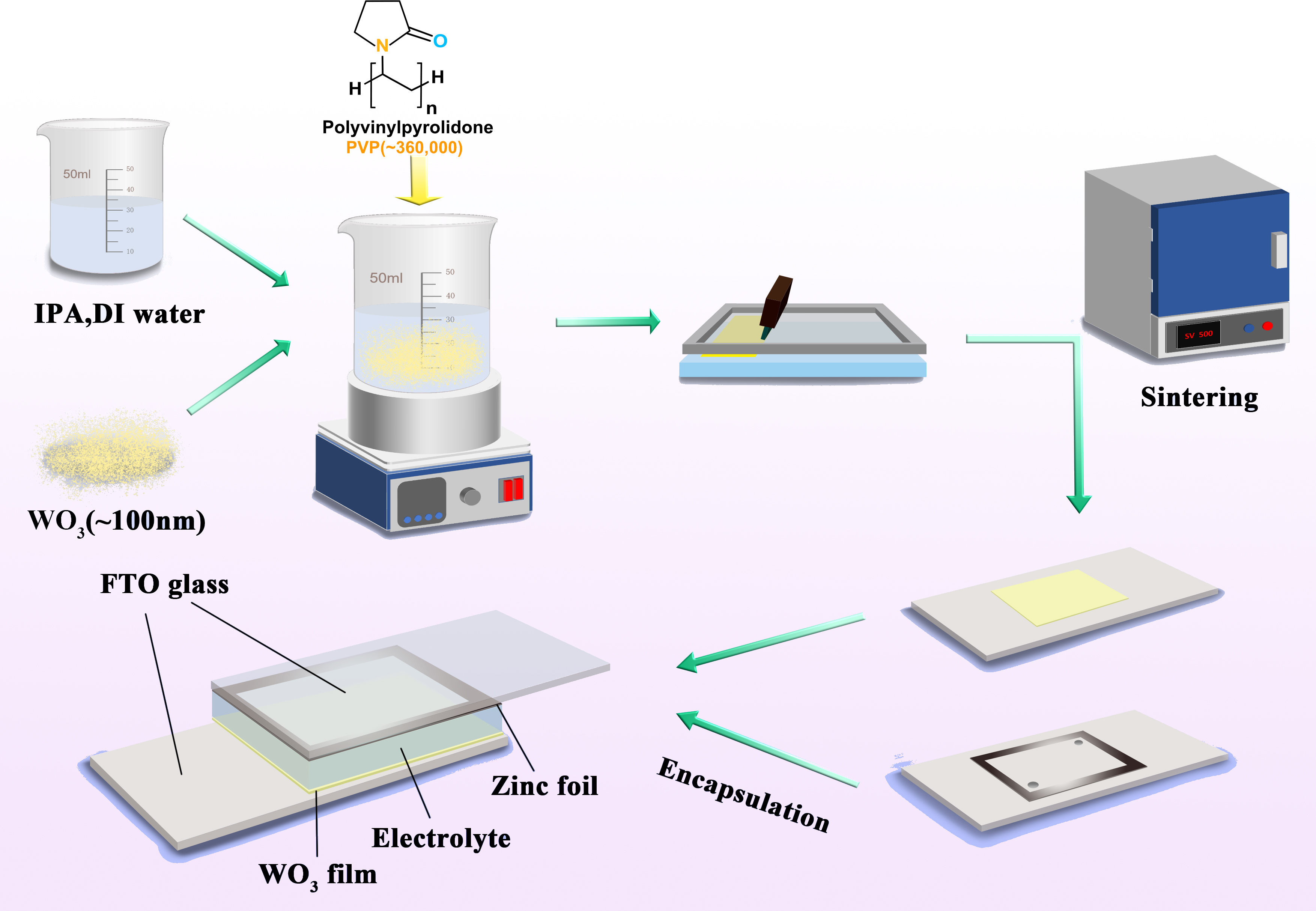
Electrochromic devices possess a sandwich-like structure and can change their color by changing their optical properties such as absorbance and transmittance through the application of voltage. It is currently a hotspot in the field of electrochemical display research and has significant applications in wearable electronics, response sensors, and flexible displays. This study uses tungsten trioxide (WO3) as the cathode color-changing material, zinc foil as anode, and a water-based ink prepared by mixing nano-WO3, solvent, and polyvinylpyrrolidone (PVP) as the thickener in a predetermined ratio to meet the viscosity, surface tension, and particle size requirements of screen printing. The WO3 ink is then transferred onto a fluorine-doped tin oxide (FTO)-coated conductive glass through screen printing, and after sintering at 500∘C, the color-changing layer is encapsulated with VHB tape to obtain an electrochromic device with two electrodes. The properties of the ink at different PVP addition amounts, the morphology of the prepared films, and the performance of the devices were characterized by laser confocal microscope, scanning electron microscope (SEM), viscosity rheometer, surface tension and contact angle tester, fiber spectrometer, and an electrochemical workstation. The results showed that when isopropanol and deionized water were used as solvents, and 8 wt.% PVP as the thickener; the electrochromic device made after sintering had good transparency and could achieve an optical modulation rate of 55.9% within 36 s. This work can significantly impact the industrial application of large-area, low-cost intelligent windows using screen printing technology.